
LD-COVID RAPID TEST FOR SYMPTOMATIC AND ASYMPTOMATIC PEOPLE
LD-CoV-2 Rapid Test
Revolutionary COVID Sensor Technology
Rapid, Accurate, and Comparable with PCR
A critical aspect of the LD-CoV-2 Rapid Test is an ultra-low limit of detection (LoD), comparable with PCR testing, allowing detection of the CoV-2 even in human breath, as well as in other specimens such as nasopharyngeal swabs, saliva, mucus, and on various surfaces even at low viral loads.
This will be achieved by utilizing nano-structured materials with a large interfacial area (500-1000 m2/g) as substrates for antibody immobilization.
Such “double nanostructures“ will provide sensitivity amplification resulting in a limit of detection of just 1000 – 10000 virion/ml, making it comparable to that of PCR testing.
As a result, there will is an expected sensitivity of at least 95%, and specificity of 99-100% with test time of only about 5-10 min.
LD-CoV-2 sampling applications
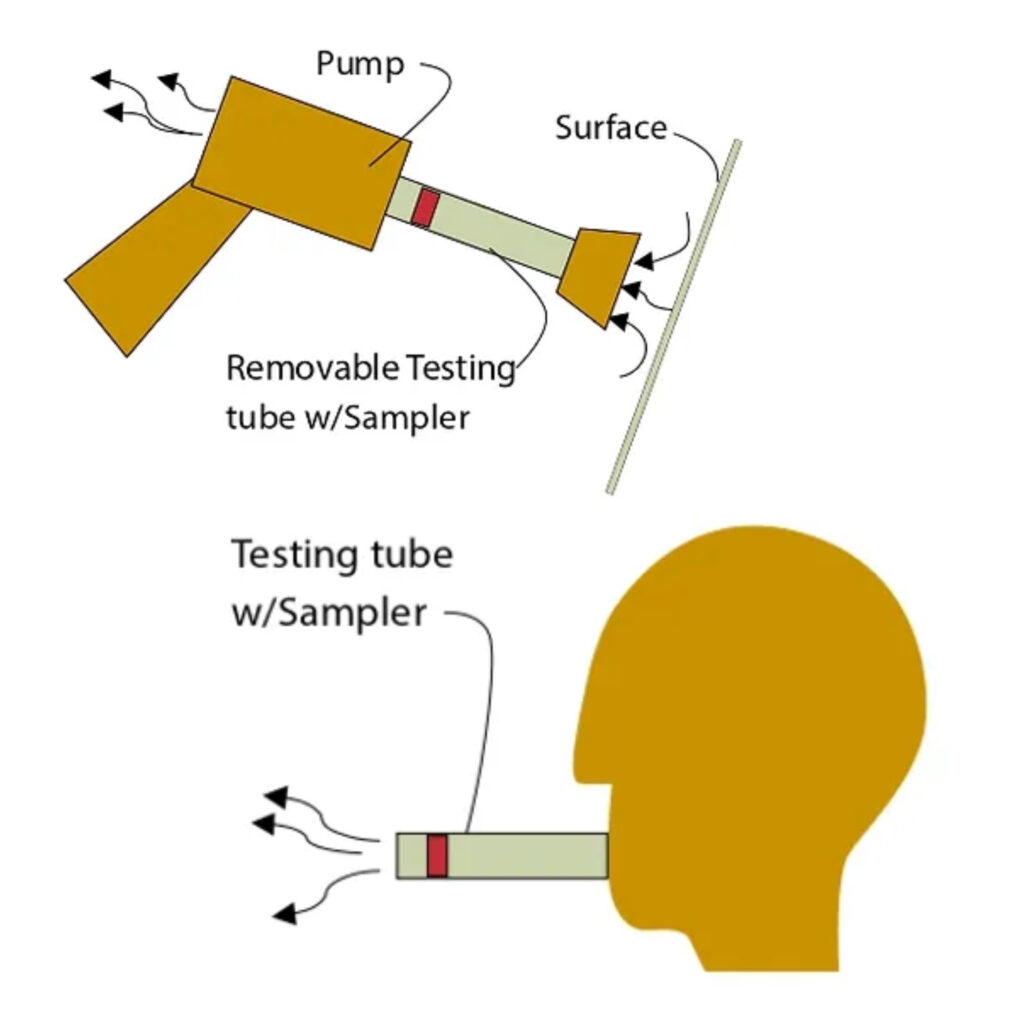

Levi Diagnostics is developing new technology which provides the first rapid (5-10 min), cost-effective ($3-5 per test cartridge), accurate test with a sensitivity comparable to PCR. In addition, it is equipped with wireless communication that allows the user to receive results on a personal smartphone/PC without the need to visit a laboratory or doctor’s office.
We named this device LD-CoV-Test.


We have preliminary data proving an extraordinary level of sensitivity of nanosilicon as a substrate for immobilization of antibodies specific to the CoV-2 virus. The graph shows a significant response of nanosilicon immunosensor to S1 protein at a 10 picogram/ml concentration. In this experiment, antibodies specific to S1 protein (spike protein of CoV-2 virus) were immobilized on the nanosilicon surface.

Critical Advantages
The developing of our ultra-sensitive immunosensors will provide critical advantages over current Sars-CoV-2 tests available on the market:
- An ability to do rapid, accurate and massive testing of asymptomatic individuals
- Non-invasive sampling method using the human breath
- Ability of sampling and detecting viruses spread over various surfaces
Comparison with existing rapid tests products on the market
The LD test has analytical sensitivity more than 1000 times higher than
commercially available rapid tests to CoV-2

*LFIA is based on the color-changing reaction.
LoD data is taken from the article https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8026170, where commercially available rapid tests were tested to SARS-CoV-2 N protein.
LD rapid test (impedance immunosensor) tested to of SARS-CoV-2 S1 protein.
COVID-19 Pandemic
The cases of COVID-19 are increasing exponentially, thus increasing government and industry initiatives to fight the virus. This will lead to a rise in the global COVID-19 detection kits market size from 2020 to 2026.
According to the research report put forth by Global Market Insights, Inc., the market size is valued at USD 3.3 billion and is expected to witness 17.3% CAGR from 2020 to 2026 potentially reaching USD 8 billion.
The diagnostic centers’ segment accounted for around 32% of the market share in 2020, according to the report.

Currently available COVID-19 Tests
Currently, most of the coronavirus tests available on the market are molecular tests (such as PCR). However, inadequate access to reagents and equipment has slowed virus detection down and impeded efforts to mitigate the viral spread.
To get the COVID-19 pandemic under control, a growing number of health and medical experts are making clear the need for additional testing for the SARS-CoV-2 virus. The most promising approach suggesting rapid and massive tests for the SARS-CoV-2 virus is based on antigen-antibody interaction (immunoassay).

A few companies have been developing these immunoassay tests, however, these have a sensitivity lower than molecular tests, which results in missing the infection at an early stage and inability to diagnose asymptomatic virus carriers.
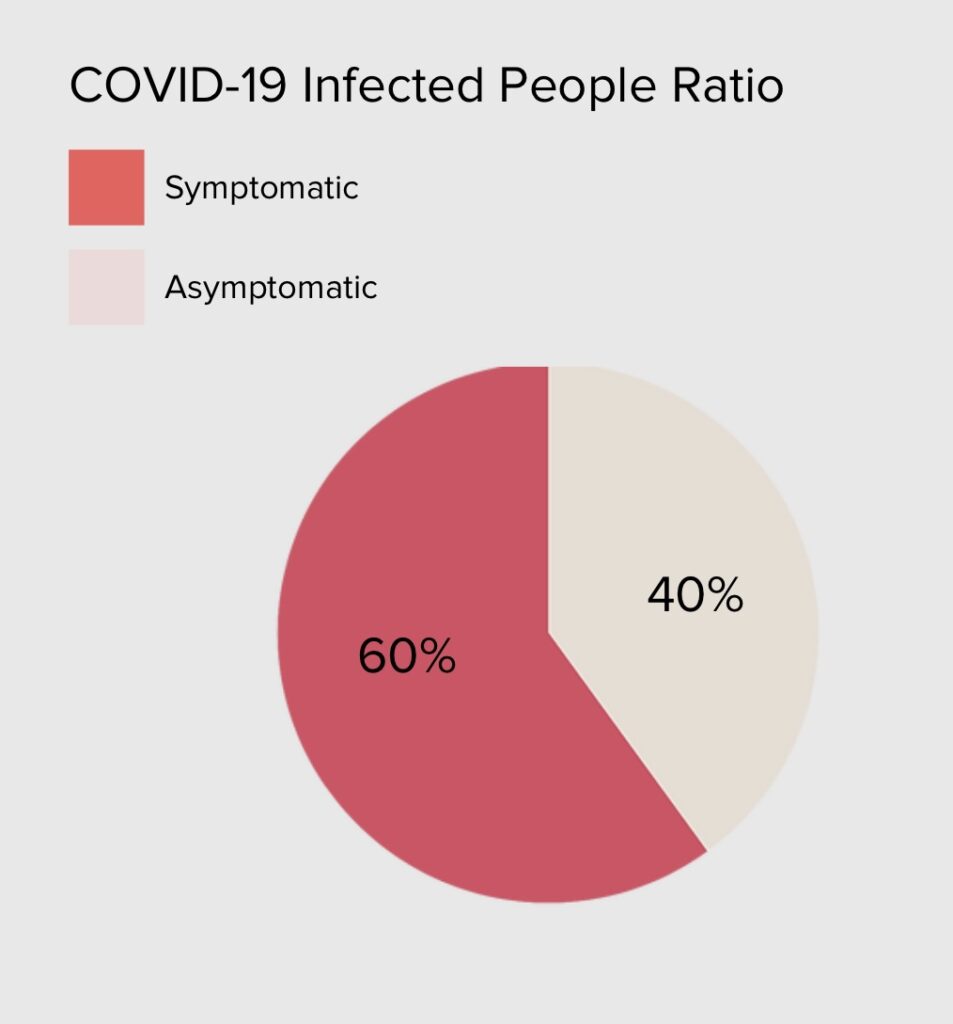
According to recent data the percentage of asymptomatic carriers is about 40% of the total number of infected people. Also, most test cartridges need to be examined by special machines installed at health provider facilities, thus preventing their use in the home environment.
Asymptomatic Spread
A large proportion of the people who contract the novel coronavirus get an asymptomatic version of COVID-19. Studies have shown that asymptomatic individuals are primarily responsible for the spread of the COVID-19 pandemic. These individuals are more likely to travel, work and socialize. The CDC estimates that 40% of infections are asymptomatic and 50% of transmissions occur before symptoms appear. Experts worry that failing to test asymptomatic carriers could not only result in more infections but also hinder contact tracing efforts. 80% of middle schoolers and teenagers do not show symptoms if they contract the coronavirus.
Levi Diagnostics will provide high-accuracy diagnosis for both symptomatic and asymptomatic people in less than 10 minutes, a revolutionary breakthrough in testing for COVID-19.